HARD
JEE Main
IMPORTANT
Earn 100
Consider the reversible isothermal expansion of an ideal gas in a closed system at two different temperatures and . The correct graphical depiction of the dependence of work done vs the final volume is:
(a)
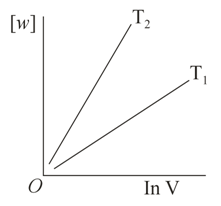
(b)
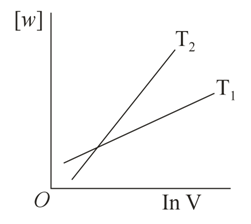
(c)
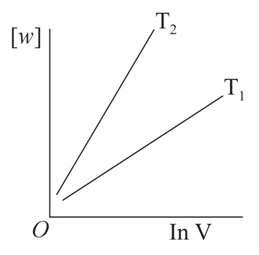
(d)
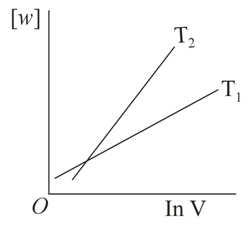
9.23% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
Among the following, the set of parameters that represents path functions, is:
i)
ii)
iii)
iv)
EASY
JEE Main
IMPORTANT
An ideal gas undergoes isothermal expansion at constant pressure. During the process:
MEDIUM
JEE Main
IMPORTANT
The standard enthalpy of formation of is . If bond enthalpy of is and that of is , the average bond enthalpy of bond in is :
MEDIUM
JEE Main
IMPORTANT
The magnitude of work done by a gas that undergoes a reversible expansion along the path shown in the figure is _________.
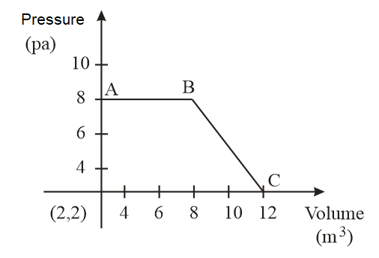
MEDIUM
JEE Main
IMPORTANT
For silver, If the temperature of moles of silver is raised from pressure, the value of will be close to:
MEDIUM
JEE Main
IMPORTANT
Which one of the following equations does not correctly represent the first law of thermodynamics for the given processes involving an ideal gas? (Assume non- expansion work is zero)
HARD
JEE Main
IMPORTANT
The standard heat of formation of ethane is , if the heat of combustion of ethane, hydrogen and graphite are and , respectively. The value of to the nearest integer is_____
MEDIUM
JEE Main
IMPORTANT
For the reaction;
at
Hence in is _____________.
